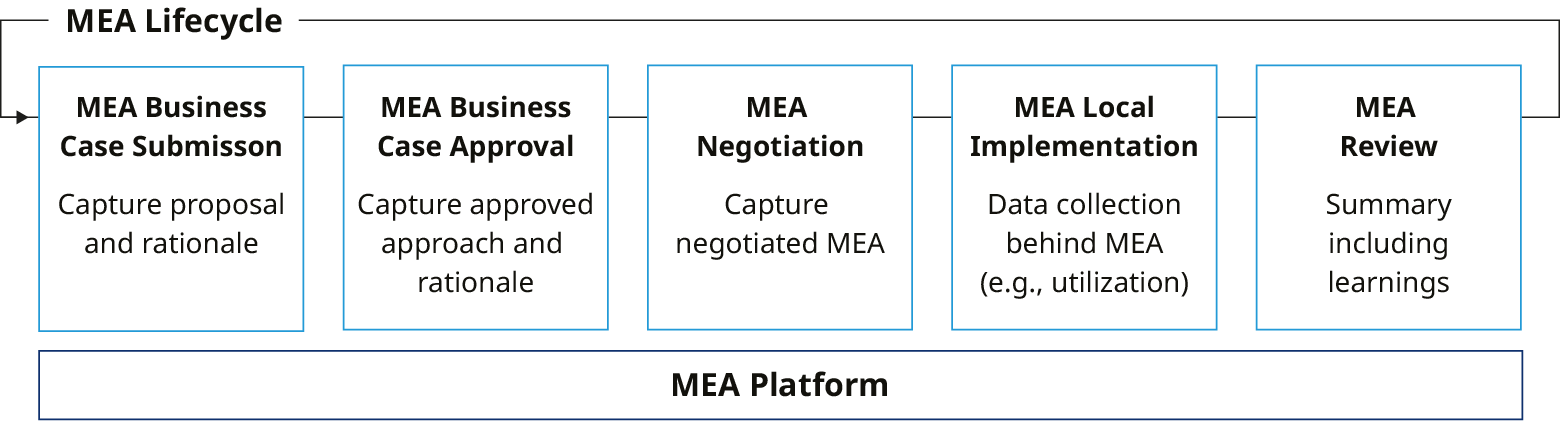
Managed entry agreements (MEAs) have become increasingly important in commercializing novel therapies, serving as a powerful means to address concerns and uncertainties about the health value and economic consequences of these treatments.

While relevant across all biopharma activity, they are of particular strategic interest in oncology, rare diseases or gene therapy, where a product’s value is promising but uncertain at launch, and evidence of real-world performance is highly desirable. The challenges of making MEAs work, especially where patient-level and outcomes data are needed, are well recognized within the industry and by payor stakeholders. While these issues remain important, biopharma organizations often overlook the internal requirements of monitoring and measuring MEA success. This white paper explores key considerations to proactively build the right organizational environment and operating model for measuring business impact of MEAs.